Introduction
Transient ischemic attack (TIA) is associated with acute infarction on diffusion-weighted imaging (DWI) in 21% to 67% of patients [
1ŌłÆ
4]. As insights had been gained in the clinical significance of DWI lesions in TIA patients, a tissue-based redefinition of TIA was proposed [
5]. According to the tissue-based definition, only TIA without evidence of acute infarction is classified as TIA. TIA associated with infarction on brain imaging is categorized as ischemic stroke (IS). However, it is debated whether TIA with infarction can be simply regarded as IS despite different clinical manifestations.
Comparison of the characteristic DWI changes between acute infarction associated with TIA and that with IS may clarify whether the pathophysiologic mechanisms are also different between them. It has been reported that the typical DWI finding of TIA with infarction is small lesion size [
2ŌłÆ
4,
6,
7]. Although the lesion size of TIA is smaller than that of IS, there is no size threshold that differentiates TIA from IS because the lesion size of TIA widely overlaps with that of small IS [
6,
8]. The factors determining whether small infarcts are clinically manifested by TIA or IS remain to be elucidated.
The author postulated that the lesion location may also play a role in the clinical manifestation of small infarcts because the lesion location is closely linked to the manifestation of neurological deficit. Since small infarcts are often found in the form of a lacunar infarct in the subcortical areas, the authors investigated whether the lesion location is different between subcortical lacunar infarcts presenting with stroke and those presenting with TIA.
Methods
This study was performed retrospectively based on the stroke registry, medical records and magnetic resonance imaging (MRI) data. The group of TIA with subcortical infarct (TSI) included consecutive patients who were admitted to Kangbuk Samsung Hospital between 2008 and 2020 with the diagnosis of TIA accompanied by a relevant infarct in the subcortical area on DWI. TIA was defined as an acute episode of neurologic deficit lasting less than 24 hours that resulted from focal cerebral ischemia. The group of lacunar stroke with subcortical infarction (LS) included patients who were admitted between 2018 and 2020 because of subcortical lacunar stroke. Lacunar stroke was defined as focal neurologic deficits persisting longer than 24 hours caused by a small (<2 cm) infarction. Because the corona radiata (CR) is the common site of subcortical lacunar infarction [
9,
10] and is the area where the lesion location can be analyzed in detail, we analyzed patients with a subcortical infarction limited to the CR.
This study was approved by the Institutional Review Board, at Kangbuk Samsung Hospital (KBSMC 202204024) and met the standards of the Declaration of Helsinki.
The patients, who underwent brain MRI (1.5-T; GE Medical Systems, Waukesha, WI, USA) including DWI within 3 days of symptom onset, were eligible for the study. DWI was obtained with a single-shot, echo-planner sequence with the following parameters: repetition time, 6,000 ms; echo time, 84 ms; b values of 0 and 1,000 sec/mm2; field of view, 26├Ś26 cm; image matrix 128├Ś128; slice thickness, 5mm; and 23 axial slices. Acute infarction responsible for the presenting neurologic deficits was identified using DWI. The DWI lesion of LS was considered to be a single, small (<2.0 cm in diameter) infarct limited to the CR adjacent to the lateral ventricle. Inclusion criteria for the lesion location of TSI were the same as that of LS.
The lesion size was defined as the largest infarct area measured with the built-in image analysis program in the picture archiving and communications system (MultiVox.NET; TechHeim, Seoul, Korea). An infarction on DWI was outlined with the guided imaging tool and the lesion area was computed automatically according to the circumscribed area (
Figure 1A).
The distance between the most lateral point of the anterior horn of the lateral ventricle (A) and the most lateral point of the posterior horn of the lateral ventricle (P) was measured (AP). The distance between the center of the lesion (L) and the most lateral point of the posterior horn of the lateral ventricle (P) was also measured (LP). The relative anteroposterior location of the lesion in the CR was determined as the LP/AP ratio (
Figure 1B). The lesion size and location were measured independently by two observers who were blind to the clinical status. The results were averaged. The interrater reliability assessed with the intraclass correlation coefficient was 0.983 (95% confidence interval [CI], 0.973ŌłÆ0.990; p<0.001) for the lesion size and 0.989 (95% CI, 0.982ŌłÆ0.993; p<0.001) for the lesion location.
Statistical analysis was performed to identify differences between patients with TSI and those with LS using t-test for the comparison of continuous variables and FisherŌĆÖs exact test for dichotomized variables. Statistical significance was established at p<0.05. IBM SPSS for Windows ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Results
During the study period, 153 patients with TIA had an acute infarction on DWI. Among them, 23 patients had an infarction limited to the CR. Forty-seven patients with IS had a lacunar infarction in the CR. Thus, 23 patients with TSI and 47 patients with LS were included in the study (
Figure 2). The baseline characteristics of the patients with TSI and LS are shown in
Table 1. There was no significant difference between the two groups with regard to age, sex, and risk factors.
All patients with TSI underwent MRI after their symptoms resolved. Recurrent episodes of TIA were observed in 14 of 23 patients (60.9%). The overall symptom duration of TSI patients was 73┬▒62 minutes (range, 15ŌłÆ200 minutes). The mean time from onset to MRI was 39.2┬▒25.2 hours for TSI and 42.6┬▒22.0 hours for LS (p=0.65).
The right:left side ratio of infarcts was 11:12 for TSI and 17:30 for LS (p=0.44). The lesion size and location in patients with TSI and LS are shown in
Table 2. The LP/AP ratio was significantly different between TSI and LS (0.48┬▒0.23 vs. 0.39┬▒0.10, p=0.02). The lesion location of LS was limited to the posterior portion (LP/AP ratio Ōēż0.6) of the CR, whereas that of TSI was distributed over the whole CR. The lesion size of TSI was smaller than that of LS (37.5┬▒26.0 mm
2 vs. 63.9┬▒33.1 mm
2, p<0.01).
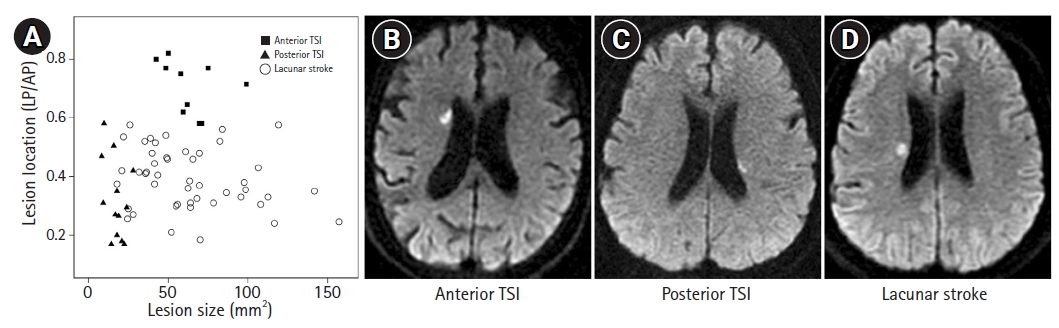
Figure 3A illustrates the scatter diagram of the lesion location and area of TSI and LS. The location-size profiles of TSI group were largely separated from those of LS group. The TSI group could be divided into two subgroups by visual inspection, the anterior and posterior group. An example case of anterior and posterior TSI, and LS is shown in
Figure 3BŌłÆ
D, respectively. The anterior TSI group was located more anteriorly than LS group (0.71┬▒0.09 vs. 0.39┬▒0.10, p<0.01) and was similar to LS in lesion size (63.5┬▒16.3 mm
2 vs. 63.9┬▒33.1 mm
2, p=0.96). The posterior TSI group had the lesion location similar to the LS group (0.32┬▒0.14 vs. 0.39┬▒0.10, p=0.10), but had the smaller lesion size than the LS group (17.4┬▒5.8 mm
2 vs. 63.9┬▒33.1 mm
2, p<0.01). Most (10/13) of infarcts in the posterior TSI group were smaller than 20 mm
2, whereas all but one infarct in the LS group were larger than 20 mm
2. Thus, the cut-off value of lesion size distinguishing IS from TIA in the posterior portion of the CR may be regarded as approximately 20 mm
2.
The demographic data including age and sex, and risk factors were not different between the anterior and posterior TSI group. Clinical profiles were also not statistically different between them, including symptom duration (81.3┬▒75.3 vs. 60.0┬▒36.9, p=0.51), recurrent episodes of TIA (5/10 vs. 9/13, p=0.42), time from onset to MRI (40.3┬▒27.6 hours vs. 38.1┬▒25.6 hours, p=0.90), presence of ipsilateral large artery disease (4/10 vs. 1/13, p=0.09).
Discussion
The results of the current study demonstrated that TSI had different lesion distribution from LS. TSI involved the whole territories of the CR, whereas LS was limited to the posterior portion of the CR. TSI also had distinct lesion location - size characteristics from LS. There were two types TSI in terms of location - size patterns. The first type of TSI was located more anteriorly than LS and had similar lesion size to LS. The second type of TSI was located in the posterior CR as was LS and had a smaller lesion size than LS.
These characteristic lesion features of TSI may be explained by the presence of a noneloquent region in the anterior CR. The motor fibers of the corticospinal tract descend through the posterior portion of the CR [
11,
12]. In contrast, the anterior portion of the CR may be related to association pathways where a small lesion may not cause apparent motor deficit. The authorŌĆÖs previous study on the location of subcortical silent or lacunar infarcts also suggested the existence of silent areas in the anterior CR [
13].
The lacune-size of infarcts occurring in the anterior noneloquent CR may cause silent or transient neurological symptoms, so called TIA, whereas infarcts in the posterior symptomatic CR should be smaller to produce TIA.
To the authorsŌĆÖ knowledge, this is the first study that identified the lesion characteristics of the location and size that clearly distinguish TIA from IS. Previous studies found that TIA had a smaller lesion size than IS, but did not clearly differentiate TIA from small IS because of wide range of size overlap [
6,
8]. The reason for these results may be that they did not consider the location of infarcts based on the topography of the eloquent and noneloquent region. Although restricted to the subcortical area, the present study revealed that an infarct size threshold that distinguishes TIA from IS was present only in the eloquent site as an area of about 20 mm
2. In the noneloquent area, the lesion size of TIA may be similar to that of IS.
The lesion size definition for lacunar infarcts in previous studies was various from <1.5 cm to <2.0 cm. Therefore, different criteria for the lesion size of lacunar infarcts may produce different results. We selected the criterion of wider range of lesion size (<2.0 cm rather than <1.5 cm) because we did not know the range of the lesion size of TSI and the size criterion of <2.0 cm for lacunar infarcts was also frequently used in MRI studies [
14,
15]. Furthermore, the lesion size was <1.5 cm in most (65/70, 92.9%) patients included in this study, which would not affect the overall results. The object of this study was to differentiate a small-sized infarct manifested by TIA from that by stroke regardless of stroke mechanism because the lesion characteristics of TSI were reportedly small lesion size and stroke etiologies have not been found as a significant factor for determining the clinical manifestation as TIA or stroke. Thus, in this study, lacunar stroke was defined as just a stroke with a single small infarction regardless of stroke etiologies. However, with regard to the stroke etiologies, patients with TSI have small vessel disease in 17/23 (73.9%) and two or more etiologies in 6/23 (26.1%) and patients with LS have small vessel disease in 43/47 (91.5%) and two or more etiologies in 4/47 (8.5%). There is a trend that two or more etiologies were more frequent in patients with TSI than in patients with LS, although it was not statistically significant (p=0.07). More sample size may be required to clarify this issue.
This study has some limitations. First, this is a retrospective study using a stroke registry and medical records. Therefore, some patients with TSI may not have been included in this study because their symptom duration was not clear. Second, the LP/AP ratio used as a localization method in this study may be influenced by an individual anatomical variation of the lateral ventricle. Nevertheless, this method reliably assessed the relative location of infarcts within the CR in the previous studies [
15,
16]. Third, the current study only included subcortical infarction limited to the CR for the purpose of lesion location analysis. Further studies including other brain areas are required for the generalization of the results of this study.
In conclusion, the present data suggest that small infarcts are manifested by lacunar stroke or TIA depending on the lesion location and size. In the eloquent area, TIA with infarction had smaller (<20 mm2) lesion size than lacunar stroke. In the noneloquent area, TIA with infarction was found to have a similar lesion size to lacunar stroke. TIA with infarction may be a unique syndrome distinguished from small IS not only in the clinical manifestations but also in the lesion characteristics. Future studies with a larger number of cases and that examine other brain areas will confirm this concept.











 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print